THE ACIDITY OF THE SOIL
Soil, as an environment for plant growth and development, may be acidic, neutral or alkaline. To explain, what does such a reaction depend on, you need to know what is known as the dissociation of water in chemistry. It is about this, that some water molecules break down into hydrogen atoms, carrying a positive electric charge (therefore they are marked with the symbol H + and called hydrogen ions), and on the so-called. hydroxide ions, with a negative electric charge and marked with the symbol OH . The predominance of positive H * hydrogen ions is the cause of the acidity of the environment, while the predominance of negative OH hydroxide ions determines its basicity, also called environmental alkalinity.
Thorough research has shown, that very few water molecules dissociate, e.g. at a temperature of 25 ° C, one liter of water contains only one ten-millionth of a gram of H and exactly the same number of gram-ions of OH . To make things easier, a shortened form of notation using the power with a negative exponent is used, and so 0,0000001 = 10 to the power -7. It is in a liter of clean water 10 to the power of -7 "acidic" ions” i 10 to the power of -7 "alkali ions."”. When determining the reaction of the environment, the concentration of hydrogen ions was taken as the measure. From the Latin name of the exponent of the concentration of hydrogen ions [Hydrogen power) this concentration is denoted by the symbol pH. Because in one pure liter (distilled) water, so in a neutral environment, is 10 to the power of -7 hydrogen ions of H +, the pH is 7. If you were to add a pinch of lime to this water, then the number of OH ions would increase , at the same time it decreased the number of H + ions, and the reaction becomes alkaline. So remember, that the neutral pH is equal 7. This indicator indicates a neutral reaction. Reaction below 7 means acidification of the environment, above 7 means alkaline reaction.
 In agricultural practice, the pH of soils is regulated by liming. In general, mineral soils should have a pH 6,0-6,5; light soils should not have a higher pH than 5,5-6,0. Medium and heavy soils should be limed like this, so that the pH is from 6,5 do 7,5. The degree of soil acidification (with some approximation) can be most easily determined as follows: take the soil on a saucer and pour a few drops of vinegar over it. If there has been a teardown, means, that the soil is rich in lime.
In agricultural practice, the pH of soils is regulated by liming. In general, mineral soils should have a pH 6,0-6,5; light soils should not have a higher pH than 5,5-6,0. Medium and heavy soils should be limed like this, so that the pH is from 6,5 do 7,5. The degree of soil acidification (with some approximation) can be most easily determined as follows: take the soil on a saucer and pour a few drops of vinegar over it. If there has been a teardown, means, that the soil is rich in lime.
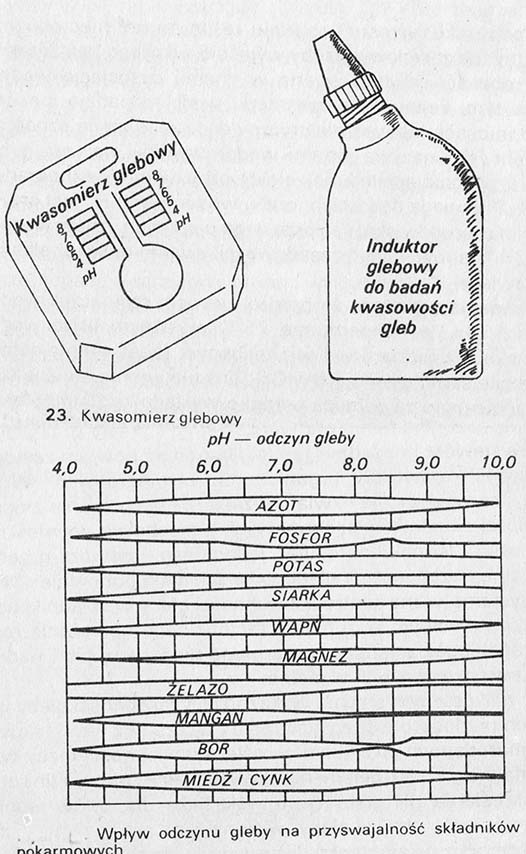
A more accurate determination of liming needs can be obtained with a soil acid meter. The Hellig acid meter is widely available. It is a very simple instrument, which consists of a plate and a bottle with an indicator. There is a color scale on the board, groove and longitudinal groove. A pinch of soil is poured into the groove and poured with the indicator. Depending on the pH of the soil, the obtained solution will have the appropriate color. The pH adjustment consists in comparing this color of the solution with the scale on the plate or on a separate color paper insert. More accurate data can be obtained, by sending the sample to a chemical-agricultural station.
The influence of soil reaction on plants is multifaceted; it can be direct and indirect. Particular groups of plants require a specific soil pH. In most cases, the indirect effect of the reaction on the plant is more important, because it mainly affects the solubility of various minerals in the soil. On acidic soils, e.g.. an excessive amount of such ingredients is formed, how: knee, iron, mangan, and therefore they become harmful to plants. The listed ingredients negatively affect both the plant roots, as well as the digestibility of nutrients.
The influence of the reaction on the digestibility of nutrients is shown in the figure. You can see from it, that reaction has the greatest influence on bioavailability: phosphorus, iron, manganu i boru. The lower the pH is, the availability of phosphorus is lower, while the other three nutrients are difficult to access when alkaline. As soil acidity increases, the activity of iron increases, clay and manganese. Under these conditions, soluble phosphates are strongly bound to these components, creating very complex and non-digestible relationships. If the soil pH exceeds significantly 7, plant nutrition is otherwise disrupted. Comprehensive are formed, insoluble calcium phosphates, which not only reduce the bioavailability of soil phosphorus, but also phosphorus fertilizers. Thus, the digestibility of nutrients depends to a large extent on the pH of the soil. A good supply of plants with nutrients increases not only the yields of vegetables and fruits, but it also affects their quality, and above all, the content of mineral compounds in them, considered by nutritionists to be the best source of mineral salts for the human body.
The pH of the soil also influences the development of plant diseases. I tak np. acid reaction promotes the development of cabbage syphilis, downy mildew of onion, a reduces the development of scab in potatoes.
